Mains Daily Question
Aug. 25, 2023
Elucidate the provisions of the Registered Medical Practitioner (Professional Conduct) Regulations, 2023. Also, highlight the challenges to the adoption of Generic Drugs in India and suggest measures to address them.
Approach:
Introduction: Introduce the context of recent guidelines published by NMC on professional conduct.
Body: Write the features of Registered Medical Practitioner (Professional Conduct) Regulations, 2023 and the challenges associated with which it is getting backlash. Suggest a suitable way forward to deal with the controversy.
Conclusion: Conclude by highlighting the need to balance the interest of all stakeholders
Answer:
The National Medical Council has recently issued guidelines mandating doctors to prescribe only generic drugs, which has been met with strong opposition from the Indian Medical Association, who called the guidelines unscientific and impractical.
Provisions of the new guidelines on professional conduct:
- Social media usage: Doctors are allowed to share accurate and verifiable information on social media platforms, but they must avoid disseminating misleading or false content. They are also prohibited from discussing patient-specific treatment details, sharing patient scans, or endorsing patient testimonials.
- Right to refuse treatment: Doctors have the right to refuse treatment to patients and their relatives who exhibit abusive, unruly, or violent behavior. They are also allowed to deny treatment to patients who cannot afford it, except in cases of medical emergencies. Discrimination based on factors such as gender, race, religion, caste, or social status is strictly prohibited.
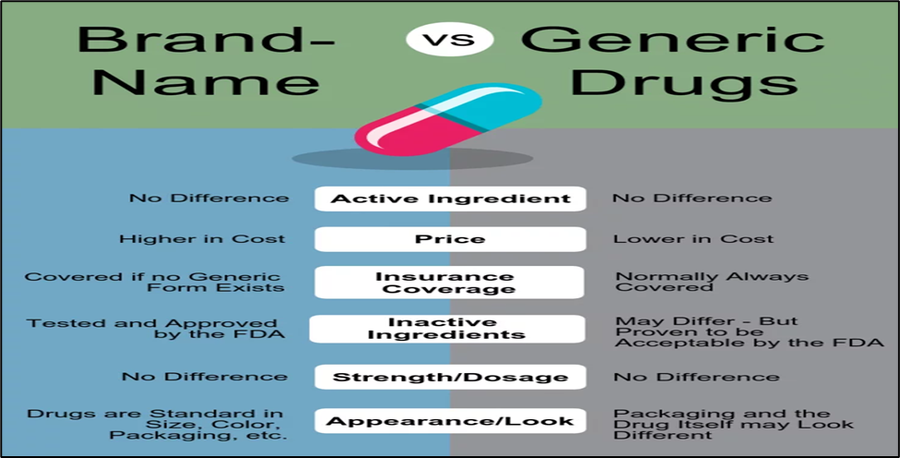
- Prescription and medication guidelines: Doctors are required to write prescriptions in clear and legible capital letters. They are also encouraged to prescribe generic medicines, promote the judicious use of fixed-dose combinations, and educate patients about the equivalence of generic and branded medicines.
- Continuous Professional Development (CPD): Medical professionals are required to engage in ongoing learning activities throughout their active years to stay updated with the latest medical advancements and practices. They are mandated to earn a minimum of 30 credit points in their relevant fields every five years as part of their CPD requirements.
- Conference participation guidelines: CPD sessions and conferences must not be sponsored by the pharmaceutical industry to ensure unbiased and evidence-based education. Medical practitioners are advised to refrain from participating in third-party educational activities that are sponsored by pharmaceutical companies. Acceptance of gifts, hospitality, cash, or grants from pharmaceutical companies is strictly prohibited.
- Other guidelines: Doctors are prohibited from soliciting patients directly or indirectly through social media platforms. They are also prohibited from practicing medicine while under the influence of alcohol or drugs.
Challenges to the adoption of Generic Drugs in India:
- Low awareness among patients and prescribers. Many patients and prescribers in India are not aware of the benefits of generic drugs. They may be more familiar with branded drugs and may believe that they are of higher quality. Example A 2019 study by the Indian Council of Medical Research found that only 20% of patients in India were aware of generic drugs.
- Negative perception of generic drugs. Some people in India have a negative perception of generic drugs, believing that they are of lower quality than branded drugs. This perception may be due to the fact that generic drugs are often cheaper than branded drugs. Example 2018 survey by the National Pharmaceutical Pricing Authority found that 60% of Indians believed that generic drugs were of lower quality than branded drugs.
- Quality issues. There have been some cases of quality problems with generic drugs in India. This is due to a number of factors, including lax regulatory oversight and poor manufacturing practices. Example In 2018, the Indian government recalled millions of doses of generic drugs after it was found that they were contaminated with bacteria.
- Lack of availability. Generic drugs may not be available in all parts of India, especially in rural areas. This is due to a number of factors, including the lack of a strong distribution network and the low profit margins for generic drugs. Example A 2019 study by the Indian Council of Medical Research found that only 60% of rural pharmacies had a stock of generic drugs.
5-Patent protection. Some brand-name drugs are still under patent protection in India, which means that generic versions of these drugs cannot be manufactured or sold. This can make generic drugs more expensive and less accessible. Example In 2023, there are still about 200 brand-name drugs that are under patent protection in India.
Way Forward:
1-The NMC and the IMA can continue to engage in dialogue to reach a mutually agreeable solution. This could involve the NMC providing more clarity on the guidelines and the IMA providing more evidence to support their objections.
2-Strengthening the regulation of drug prices and ensuring the quality of generic medicines can build trust among patients. The National Pharmaceutical Pricing Authority (NPPA) is responsible for regulating the prices of pharmaceutical products in India.
3-Enhancing affordability: Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP) is a scheme that aims to provide quality generic medicines at affordable prices through dedicated Jan Aushadhi stores.
4-Patients could be more educated about the benefits of generic drugs. This could help to reduce the demand for branded drugs and make it easier for doctors to prescribe generics.
5-The pharmaceutical industry could play a more active role in promoting the use of generic drugs. This could involve providing financial incentives to doctors and patients to use generics.
Hence the best approach will be the one that best balances the interests of patients, doctors, and the pharmaceutical industry.